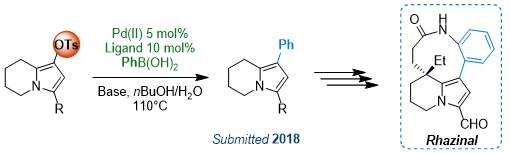
Palladium Catalyzed Cross-Coupling Reactions are nowadays essential to organic chemistry, allowing to conveniently build up complex molecules through C-C bond formation. These reactions usually involved two partners: a vinyl or aryl halide or triflate and different organometallic reagents, such as boronic acids or esters (Suzuki-Miyaura reaction), organotins (Stille reaction) and others, or alkenes (Mirozoki-Heck reaction) or alkynes (Sonogashira reaction). However, limitations still exist regarding the conditions but also the starting material nature. Iodides and bromides are more reactive but for industrial and cost reasons, chlorides can now be used, although they required harsher conditions and/or specific catalysts. Triflates usually are as reactive as iodides, but their introduction through triflic anhydride requires specific conditions due to the sensitivity of this reagent. However, other inexpensive sulfonates such as nosylate and tosylate derivatives can offer a convenient alternative to traditional halide or triflate partners.
Aryl or Vinyl Nosylates as Versatile Partners
For the first time, we have demonstrated that para-nitrophenylsulfonate (nosyl; Ns) group act as a chip and excellent partner in various Pd-catalyzed cross-coupling reactions.
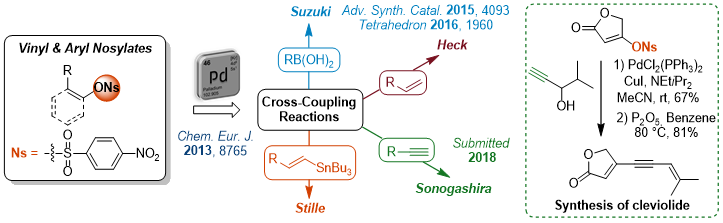
Tosylates as Convenient Partners for Heteroaryl Derivatives Synthesis
We are also working on the development of efficient conditions for unprecedented Suzuki-Miyaura cross-coupling reactions between heteroaryl tosylates and boronic acids to achieve the total synthesis of Rhazinal alkaloid.